| Home | Energy | Nuclear | Electricity | Climate Change | Lighting Control | Contacts | Links |
|---|
INTRODUCTION:
The CO2 retention time Tr is the average time that a CO2 molecule spends in Earth's atmosphere before it is absorbed by the ocean. The CO2 retention time can be calculated from C-14 concentration decay data acquired subsequent to atmospheric nuclear bomb tests. The CO2 retention time in combination with atmospheric CO2 concentration data from Mona Loa allows calculation of the total rate at which oxidation of fossil carbon injects CO2 into Earth's atmosphere.
Typically this data shows that the total flux of fossil CO2 into the atmosphere is about 1.87X the rate directly indicated by governmental reports relating to combustion of fossil fuels. The discrepency is because governmental reports only address to the portion of the fossil CO2 flux into the atmosphere for which governments collect royalties or taxes.
If N carbon-14 (C-14) atoms bound to oxygen as CO2 molecules are injected into the atmosphere the retention time Tr is given by:
Tr = [N / (- dN / dT)].
This web page presents a quantitative analysis of the atmospheric carbon dioxide (CO2) retention time Tr and the effect of Tr on the computed rate of fossil CO2 injection into the atmosphere.
If a nuclear bomb was the only source of C-14 in the atmosphere then:
(dN / dT) = (- N / Tr)
which has a solution:
N = No exp[(T - To) / Tr]
or
dN / dT = (- 1 / Tr) No exp[- (T - To) / Tr]
= (-N / Tr)
or
Tr = [N / (- dN / dT)]
as expected.
If due to cosmic rays and nuclear reactors there is a constant injection source Ri of new C-14 tagged CO2 then:
dN / dT = (- N / Tr) + Ri
dN / dT = 0
at which time the final value:
N = Nf
is given by:
Nf = Ri Tr
Try solution:
(N - Nf) = (No - Nf) exp[(T - To) / Tr]
which gives:
dN / dT = (N - Nf)(- 1 / Tr)
= (- N / Tr) + (Nf / Tr)
= (- N / Tr) + Ri
Hence from experimental C-14 atmospheric concentration data we can evaluate Tr using the equation:
N = Nf + (No - Nf) exp[(T - To) / Tr]
C-14 is formed in Earth's atmosphere by cosmic rays, nuclear reactors and by nuclear explosions. C-14 naturally decays to N-14 by electron emission with a half life of 5730 years. However, in Earth's atmosphere the C-14 concentration spikes produced by nuclear explosions diminish with an apparent half life of ~ 11 years. This apparent rapid concentration diminishment rate is because atmospheric CO2 is absorbed by sea water.
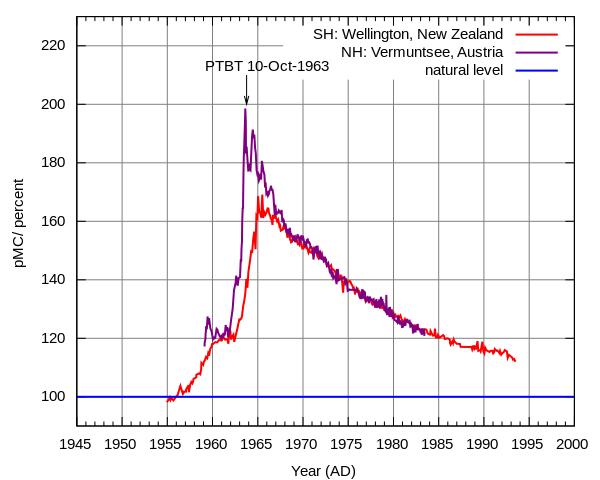
Nuclear bomb tests yielded the following graphs of atmospheric C-14 concentration as a function of time. Note that there is a time delay of about a year between the C-14 rise time in the northern hemisphere and the corresponding rise time in the southern hemisphere. Hence data taken during the first few years after the explosions is invalid due to C-14 still being in the process of spreading evenly around the world.
In order to evaluate Tr we must use data where the experimental measurements in the northern and southern hemispheres co-incide.

From this graph:
Natural Level = Nf = 100
In 1968: N - Nf = 158 - 100 = 58
In 1985: N - Nf = 120 - 100 = 20
giving:
Tr = 17 years / [Ln(58 / 20)]
= 15.966 years
The corresponding half life Th in the atmosphere is given by:
exp(- Th / Tr) = (1 / 2)
or
exp(Th / Tr) = 2
or
Th = Tr Ln(2)
= 15.966 years (0.693147)
= 11.0667 years.
CO2 BALANCE IN ATMOSPHERE:
Rx values are numbers of molecules of CO2 per unit time
Na = number of CO2 molecules in atmosphere prior to combustion of fossil fuels
Nb = current number of CO2 molecules in atmosphere
V = effective volume of atmosphere
(Na / V) = historic concentration of CO2 at sea level before fossil fuel injection, Na = constant
(Nb / V) = current concentration of CO2 at sea level after fossil fuel injection
CO2 Injection Sources:
Rt = rate of CO2 molecules entering atmosphere due to Charles Law
Re = rate of CO2 molecular emission from ocean due to solar induced evaporation
Rc = rate of CO2 injection into atmosphere due to combustion of fossil fuels
Rd = rate of CO2 injection into atmosphere due to biomatter decay
CO2 Absorption Sinks:
Ra = historic rate of CO2 molecules absorbed by ocean
Rb = current rate of CO2 molecules absorbed by ocean
Rp = rate of CO2 absorption by plants
Historically:
Rt + Re + Rd = Ra + Rp
where:
Ra = K (Na / V)
where:
K is an absorption rate constant
or
Na / Ra = (V / K)
Currently:
Rb = K (Nb / V)
and
Rs = dNb / dT = d(Nb – Na) / dT
Rt + Re + Rd + Rc = Rb + Rs + Rp
or
(Rt + Re + Rd) + Rc = Rb + Rs + Rp
or
(Ra + Rp) + Rc = Rb + Rs + Rp
or
Ra + Rc = Rb + Rs
or
Rc = (Rb - Ra) + Rs
= [(K / V) (Nb - Na)] + d(Nb - Na) / dT
If Rc = 0 then:
d(Nb - Na) / dT = - [(K / V) (Nb - Na)]
which has solution:
(Nb - Na) = [Nb - Na)|T = To] exp[- (T - To) (K / V)]
Thus:
Tr = (V / K)
Hence:
Rc = [(Nb - Na) / Tr] + d(Nb - Na) / dT
EVALUATION:
From C-14 decay time constant of C-14 in atmosphere is:
Tr = 15.966 years
Rg = CO2 emission ratre as indicated by published fossil fuel records
Rc = Rg Fx
where:
Fx > 1 = correction factor to include fossil carbon extraction not captured by published fossil fuel records
From Mona Loa data for early 2017:
Nb = 406 ppmv
and
Na = 280 ppmv
and
d(Nb - Na) / dT = 2.5 ppmv / year
Recall that:
Rc = [(Nb - Na) / Tr] + d(Nb - Na) / dT
= [(Nb – Na) / 15.966 years] + d(Nb - Na) / dT
In 2017:
Rc = [(406 ppmv - 280 ppmv) / 15.966 years] + 2.5 ppmv / year
= 7.892 ppmv / year + 2.5 ppmv / year
= 10.392 ppmv / year
From the web page titled: CARBON DIOXIDE when the atmospheric CO2 concentration was 280 ppmv the mass of CO2 in the atmosphere was about 224.91 X 10^10 tonnes.
Hence the injected mass of CO2 corresponding to an atmospheric CO2 concentration of 10.392 ppmv is given by:
[(224.91 X 10^10 tonnes CO2) / 280 ppmv] X 10.392 ppmv / year
= 8.3474 X 10^10 tonnes CO2 / year
From the web page titled: CARBON DIOXIDE for the period 2004 - 2009 inclusive:
Rc = 2.01 ppm / year + [(384.8 ppmv -280 ppmv) / 15.966 years]
= 2.01 + 6.564 ppmv / year
= 8.574 ppmv / year
The corresponding injected mass of CO2 produced is given by:
[(224.91 X 10^10 tonnes CO2) / 280 ppmv] X 8.574 ppmv / year
= 6.8871 X 10^10 tonnes CO2 / year
From the web page titled: CARBON DIOXIDE government claimed CO2 production Rg from coal, oil and natural gas in 2004 to 2009 is:
Rg = 18.43497 Fx X 10^10 tonnes CO2 / 5 years
= 3.68699 X 10^10 tonnes CO2 / year
Hence for the period 2004 to 2009:
Fx = (Rc / Rg)
= 6.8871 / 3.68699
= 1.868
~ 1.87
Hence the total fossil carbon emissions to the atmosphere during the period 2005 to 2009 was about 87% higher than indicated by the government reported amounts of coal, oil and natural gas.
Governments only reliably report on coal, oil and natural gas for which tax or royalties are collected.
SOURCES OF FOSSIL CARBON DIOXIDE NOT INCLUDED IN MOST GOVERNMENTAL FOSSIL FUEL REPORTS:
CO2 and methane emitted during muskeg melting
Steam generation via combustion of heavy oil on heavy oil extraction sites
Flared natural gas
Upstream natural gas and petroleum processing
Pipeline pumping energy
Refinery energy (typically 17% of refinery input)
Resin feed stock (goop for making vehicle tires)
Asphalt (typically 12% of refinery output used for roads, shingles, roofing, basement water sealing)
Coke (used for steel and cement production)
Electricity producers that mine their own coal but report only electricity production
Illicit and unreported oil
Illicit and unreported coal
The conclusion of this web page is that the total fossil CO2 emissions to the atmosphere are about 87% higher than indicated by published coal, oil and natural gas production reports. Hence for nuclear power to fully displace fossil fuels in Ontario instead of 7X the present functional nuclear reactor capacity more than 10X the present functional nuclear reactor capacity will be required.
This web page last updated March 18, 2017.
| Home | Energy | Nuclear | Electricity | Climate Change | Lighting Control | Contacts | Links |
|---|